Clinicians, sponsors, ethics review committees, and others are charged with ensuring that risk is in a favourable balance with benefit when patients enrol in trials. Yet how do they make this judgment, when the only evidence available is from preclinical animal studies?
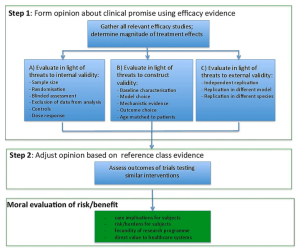
In our recent article published in the Journal of Medical Ethics1, we offer an answer to this question. We argue, first, that review committees and clinicians should evaluate the clinical promise of a new intervention based on preclinical efficacy evidence. Specifically, they should form judgments about the clinical promise of a new intervention based on how well preclinical studies address common threats to clinical generalization. Second, review committees should adjust their estimates of clinical promise based on clinical trials with related drugs (e.g. within the same class of agents). All else being equal, the stronger the clinical promise, the stronger the moral justification for embarking on an early-phase clinical trial.
Before consuming scarce financial and human resources, including exposing volunteers and patients to potentially ineffective treatments in trials, researchers should capitalize on the knowledge gained from animal efficacy studies. Ethics review committees and clinical investigators will likely protest they lack the expertise, skill or time to evaluate preclinical evidence. Or, that drug regulators like FDA already make such judgments.
However, the FDA itself states that “lack of… potential effectiveness information should not generally be a reason for a Phase 1 IND to be placed on clinical hold2,” and it explicitly delegates judgments about risk/benefit to IRBs3. This means that, if ethics reviewers and investigators really do lack the expertise, skill or time to review preclinical evidence- they are surrendering their mandate to protect patients from undue risk in early phase trials.
- Kimmelman, J. and Henderson, V. Assessing risk/benefit for trials using preclinical evidence: a proposal. J Med Ethics Published Online First: 13 October 2015 doi:10.1136/medethics-2015-102882
- Guidance for Industry: Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well-Characterized, Therapeutic, Biotechnology-derived Products. In: Services USDoH, ed., 1995.
- 46 FR 8975, Jan. 27, 1981, as amended at 56 FR 28029, June 18, 1991; 66 FR 20599, Apr. 24, 2001.
BibTeX
@Manual{stream2015-904,
title = {Balancing the Evidence: Animal efficacy studies should have more weight in the risk/benefit calculus ahead of clinical trials},
journal = {STREAM research},
author = {Valerie Henderson},
address = {Montreal, Canada},
date = 2015,
month = nov,
day = 5,
url = {https://www.translationalethics.com/2015/11/05/balancing-the-evidence-animal-efficacy-studies-should-have-more-weight-in-the-riskbenefit-calculus-ahead-of-clinical-trials/}
}
MLA
Valerie Henderson. "Balancing the Evidence: Animal efficacy studies should have more weight in the risk/benefit calculus ahead of clinical trials" Web blog post. STREAM research. 05 Nov 2015. Web. 26 Apr 2025. <https://www.translationalethics.com/2015/11/05/balancing-the-evidence-animal-efficacy-studies-should-have-more-weight-in-the-riskbenefit-calculus-ahead-of-clinical-trials/>
APA
Valerie Henderson. (2015, Nov 05). Balancing the Evidence: Animal efficacy studies should have more weight in the risk/benefit calculus ahead of clinical trials [Web log post]. Retrieved from https://www.translationalethics.com/2015/11/05/balancing-the-evidence-animal-efficacy-studies-should-have-more-weight-in-the-riskbenefit-calculus-ahead-of-clinical-trials/